Leber Congenital Amaurosis (LCA) is an inherited eye disease that affects roughly 1 in 40,000 newborns. This eye condition causes severe vision loss or even blindness right from birth or early infancy.
It’s caused by problems with the retina, the part at the back of the eye that senses light. Mutations or changes in various genes responsible for the retina’s growth and function can lead to LCA.
Gene therapy, a revolutionary medical approach, can potentially treat diseases by introducing new genes into cells. This therapy could potentially fix the genetic issues causing LCA, helping restore vision in people affected.
Gene therapy has already shown promise in treating some forms of LCA, and the FDA has approved it for a type of LCA caused by a specific gene mutation.
Let’s find out in detail!
Quick Overview:
Leber Congenital Amaurosis (LCA) is a rare genetic eye disease caused by mutations in genes crucial for retinal development and function.
Gene therapy, which involves introducing new genes into cells, could correct genetic issues causing LCA, offering a chance to restore vision.
The FDA has approved gene therapy for a specific LCA type caused by the RPE65 gene mutation, showcasing its promise in treating certain forms of LCA.
Keep reading to understand the complexities of LCA’s genetics, symptoms, and the groundbreaking role of gene therapy in potentially treating this challenging condition.
What Is LCA?
Leber Congenital Amaurosis (LCA) is a group of hereditary retinal diseases characterized by severe visual impairment from birth. This condition primarily affects the retina, the part of the eye responsible for capturing light and sending visual signals to the brain. Individuals with LCA typically experience symptoms such as early-onset vision loss, involuntary eye movements (nystagmus), diminished pupil reactions to light, and sensitivity to light (photophobia). The condition often leads to other vision complications, including extreme farsightedness (high hyperopia).
Genetics of LCA
Leber Congenital Amaurosis (LCA) is a genetic disorder causing early-onset blindness, passed through autosomal recessive inheritance. For a child to develop LCA, both parents must carry and pass on the mutated gene. Consequently, there’s a 25% chance that their offspring will inherit LCA, with each parent contributing one copy of the mutated gene. However, some forms can be autosomal dominant where a single parent can also pass on the disease to 50% of the kids.
LCA results from changes in around 28 different genes. These genes are involved in:
- The creation of cells that detect light and convert it into signals for the brain.
- The maintenance of cilia, which are tiny hair-like structures on these cells that help transport molecules and proteins.
- The refreshment of a molecule that helps these cells respond to light.
- The creation of a molecule that helps maintain retina levels.
When these genes have mutations, it can cause the cells that detect light to function poorly or degenerate, leading to severe vision loss or blindness.
Most important genes include:
| Gene | Inheritance Pattern | % of LCA Cases |
|---|---|---|
| GUCY2D | Autosomal Recessive | ~20% |
| RPE65 | Autosomal Recessive | ~6-16% |
| CRB1 | Autosomal Recessive | ~9-13% |
| RPGRIP1 | Autosomal Recessive | <10% |
| AIPL1 | Autosomal Recessive | ~5% |
| LCA5 (Lebercilin) | Autosomal Recessive | ~5% |
| CEP290 | Autosomal Recessive | ~15-20% |
| CRX | Autosomal Dominant | Rare |
| RDH12 | Autosomal Recessive | ~3-6% |
| IMPDH1 | Autosomal Dominant | Rare |
| OTX2 | Autosomal Dominant | Rare |
| SPATA7 | Autosomal Recessive | <5% |
| RD3 | Autosomal Recessive | <5% |
| IQCB1 (NPHP5) | Autosomal Recessive | Rare |
| MERTK | Autosomal Recessive | Rare |
| TULP1 | Autosomal Recessive | ~5% |
Symptoms of LCA:
Common early signs of LCA include:
- involuntary eye movements (nystagmus)
- weak or no pupil response to light
- drastically reduced visual sharpness
- light sensitivity (photophobia)
- eye poking or rubbing (Franceschetti’s oculodigital sign).
Some people with LCA might also have other eye conditions, such as cataracts (cloudy lens), keratoconus (thinning and bulging of the cornea), or glaucoma (increased pressure inside the eye).
Gene Therapy
Gene therapy is a medical technique that uses genes to treat or cure diseases. It can:
- Replace a faulty or missing gene with a normal or modified version.
- Add a new gene that can perform the function or create a beneficial protein.
- Silence or block a harmful gene causing disease.
Gene therapy can be delivered using different methods, such as:
- Viral vectors, which are altered viruses that can carry genes into cells without causing an infection.
- Non-viral vectors, synthetic molecules or particles that can carry genes into cells.
- Physical methods like injections, applying electric pulses (electroporation), or using tiny needles (microinjection).
Gene therapy has been tested for various diseases like cancer, immune disorders, metabolic disorders, neurological disorders, and inherited diseases. In some cases, it has shown promising results and gained FDA approval for conditions like spinal muscular atrophy (SMA), hemophilia A, and a type of LCA caused by RPE65 gene mutations.
Gene Therapy for LCA
Gene therapy for Leber Congenital Amaurosis (LCA) involves repairing or replacing malfunctioning genes responsible for vision loss. In LCA, specific genes crucial for retinal health are defective, impairing vision. To address this, gene therapy uses modified viruses, such as the adeno-associated virus (AAV), as delivery vehicles. These engineered viruses are safe and designed to transport therapeutic genes directly to the affected retinal cells. By injecting these viral vectors into the eye’s subretinal space, they efficiently transfer the corrective genes to the retina, offering a potential restoration of vision or halting the progression of LCA.
This treatment has been tested in clinical trials for several forms of LCA caused by mutations in a variety of genes (like RPE65, GUCY2D, CEP290, AIPL1, RDH12, LRAT, and CRB1). The results have shown that gene therapy can improve vision in some people with LCA, particularly those with RPE65 mutations. It can also slow down or even stop further loss of vision in some individuals with LCA.
The only gene therapy approved by the FDA for LCA is voretigene neparvovec (also known as Luxturna). This viral vector carries a normal version of the RPE65 gene. It was given the green light in 2017 for use in people diagnosed with retinal dystrophy caused by certain RPE65 mutations. Studies have shown that it can enhance visual function and quality of life in individuals with LCA due to these mutations.
However, there are some hurdles and drawbacks with gene therapy for LCA, such as:
- We’re still not sure about the safety and long-term effects of gene therapy.
- It might not work for everyone with LCA or might not completely restore normal vision.
- It may not work effectively for people with advanced retinal degeneration or scarring.
- It might not be accessible or affordable for everyone who needs it.
- It might not be capable of treating all types of LCA or all faulty genes involved in LCA.
So, we need to continue researching and developing gene therapy for LCA to make it more accessible, affordable, and effective for people with this condition.
An Amazing Success: Luxturna
Luxturna is an impressive, cutting-edge gene therapy product that can enhance the sight of individuals with an uncommon inherited eye disorder linked to errors in the RPE65 gene. This gene is involved in creating a protein vital for the retina, which is a thin layer at the back of the eye transforming light into brain signals.
Those with faults in their two copies of the RPE65 gene suffer from poor sight from the time they’re born or from early childhood, and they might eventually become entirely blind.
Luxturna is the first gene therapy product that the FDA has approved for this illness, offering a ray of hope for many patients who have no alternative treatments.
How Luxturna Works
Luxturna functions by providing a working copy of the RPE65 gene to cells in the retina. It uses a harmless modified virus known as adeno-associated virus serotype 2 (AAV2) as a vehicle to transport this gene.
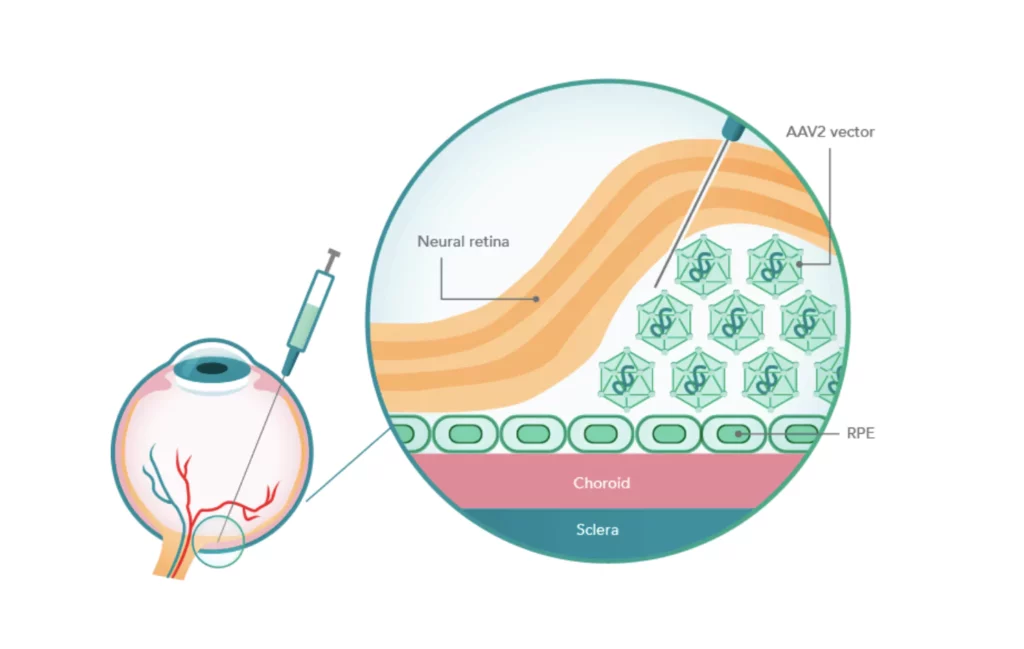
This virus is designed to carry the RPE65 gene and only infect the retina’s cells, doing no harm in the process. When the gene is inside the cells, it instructs them to produce the missing protein, restoring the visual cycle.
Luxturna is made to be a one-time treatment that can have long-term benefits on a patient’s vision.
The Process of Giving Luxturna and Its Clinical Trial Outcomes
Luxturna is given through a subretinal injection, which means it’s directly injected under the retina through a tiny opening in the eye. A professional surgeon performs this procedure under general anesthesia, and it takes approximately an hour for each eye.
The injections for each eye are done separately, typically on separate days. Patients must take certain precautions before and after the procedure, such as avoiding air travel, high altitudes, or scuba diving until the air bubble formed in the eye has vanished.
Luxturna’s safety and effectiveness were examined in a clinical trial that included 41 patients with confirmed RPE65 gene mutations and enough healthy retinal cells. The trial compared Luxturna with a control group that didn’t receive any treatment.
The primary metric was the change in functional vision, evaluated by a standardized test known as multi-luminance mobility test (MLMT). This test assesses how well patients can navigate an obstacle course under varying levels of light.
The results demonstrated that those who received Luxturna had significant improvements in their functional vision compared to those who did not receive Luxturna. These enhancements were observed as soon as 30 days after the injection and remained for at least three years.
Safety and Effectiveness of Luxturna
Luxturna is generally well tolerated by patients, but it might cause some side effects during or after its administration. The most common side effects are redness in the eye, cataract, increased eye pressure, retinal tear, thinning of the corneal stroma (dellen), a small break in the center of the retina (macular hole), accumulation of material under the retina (subretinal deposits), eye inflammation, eye irritation, eye pain, and visual disturbances.
These side effects are usually mild to moderate and can be handled with medication or surgery if necessary. There’s also a risk of serious eye infections or permanent vision loss due to complications from the procedure or gene therapy. Therefore, it’s important for patients to keep regular check-ups with their healthcare professionals and report any signs of infection or changes in vision.
While Luxturna is not a cure for RPE65-linked inherited retinal disease, it can greatly enhance the quality of life of those affected by this condition. Luxturna can assist patients to see better in dim-light conditions, do daily tasks more independently, and have more social interactions.
Luxturna can also slow down or prevent further vision loss caused by ongoing degeneration of the retina. However, Luxturna doesn’t restore normal vision or fix other vision issues such as refractive errors or astigmatism. Patients may still need glasses or other visual aids after receiving Luxturna.
Luxturna stands as a trailblazing success in the field of gene therapy and inherited retinal disease. It’s the first product of its kind to be approved by the FDA. It also highlights how genetic testing can identify patients who are eligible for this treatment and how personalized medicine can provide tailored solutions for rare diseases.
Future of LCA Gene Therapy Beyond Luxturna
Luxturna is the first therapy that uses genes to treat LCA, approved by the FDA. This treatment helps children who have issues in both copies of a gene called RPE65.
However, Luxturna isn’t a complete cure for LCA, and it doesn’t work for everyone. There are many other genes that can cause LCA, and each one may need a different gene therapy approach. Thankfully, research is currently being done to develop gene therapies for other genes associated with LCA, such as CEP290, RDH12, AIPL1, CRB1, and more.
Some of these new gene therapies have shown good early results in animals and in human trials. For example, a therapy called EDIT-101 uses a tool known as CRISPR/Cas9 to correct a specific mutation in the CEP290 gene. This mistake is one of the most common causes of LCA and affects about 20% of patients. EDIT-101 has been tested in a phase 1/2 clinical trial and has shown it can safely improve vision.
Another therapy is ADVM-022, which delivers a normal copy of the RDH12 gene to the eye cells using a different virus than Luxturna. Changes in the RDH12 gene cause a severe form of LCA that leads to total blindness by early adulthood. The FDA has granted ADVM-022 orphan drug designation and fast-track status and is currently being tested in a phase 1/2 clinical trial.
Despite this progress, there are still challenges and risks in creating new gene therapies for LCA. Some of these include:
- We don’t fully know the long-term safety of gene therapies.
- The body’s immune system may respond to the virus or the new gene and cause inflammation or rejection.
- Delivering gene therapies to the eye may need procedures like injections or surgery.
- The cost and availability of gene therapies may limit who can get them.
Ethical, Legal, and Social Considerations
Gene therapy is a new and powerful technology that can offer hope for patients with incurable diseases like LCA. However, it also brings up ethical, legal, and social questions that researchers, regulators, doctors, patients, and society need to consider.
Some of the ethical issues surrounding gene therapy include:
- Respecting the choice and informed consent of patients who take part in gene therapy trials or receive gene therapy treatments.
- Striking a balance between doing good and avoiding harm to patients.
- Ensuring equal access and opportunity for all patients who need gene therapy.
- The potential effects of gene therapy, which can modify the genes of future generations.
Some legal issues and policy challenges regarding gene therapy include:
- Regulation and oversight of gene therapy research and development by agencies like the FDA and NIH.
- Protection of intellectual property rights and patents for gene therapy products and technologies.
- Responsibility and compensation for potential harms caused by gene therapy.
- Aligning international standards and guidelines for gene therapy.
The social impact of gene therapy includes issues such as:
- Educating the public and stakeholders about the benefits and risks of gene therapy.
- Involving patients and communities in gene therapy decision-making and advocacy.
- Respecting diversity and cultural values in relation to gene therapy preferences and expectations.
- The potential stigma or discrimination faced by patients who receive or do not receive gene therapy.
Final Thoughts
Gene therapy is an emerging field that has made significant progress in treating LCA, a rare genetic disorder that causes blindness. Luxturna is the first approved gene therapy for LCA caused by RPE65 gene changes, but it’s not the only one. Other gene therapies are being developed for other genes that cause LCA, such as CEP290, RDH12, AIPL1, CRB1, and more. These gene therapies have shown promising results in preclinical and clinical studies and may provide new options for patients with LCA.
However, gene therapy is not without its challenges and limitations. There’s still a lot we don’t know about gene therapy’s long-term safety and effectiveness, as well as possible reactions or complications from the immune system. The cost and availability of gene therapy may create barriers for patients who need them.
Gene therapy is a promising but complex technology that can transform the lives of patients with LCA. It needs careful evaluation and regulation to ensure its safety, effectiveness, and ethical and social acceptability. Gene therapy is not a magic cure-all but a powerful tool that can offer hope for patients with LCA.
References:
- https://pubmed.ncbi.nlm.nih.gov/30578499
- https://my.clevelandclinic.org/health/diseases/24167-lebers-congenital-amaurosis
- https://eyewiki.org/Leber_Congenital_Amaurosis
- https://www.nih.gov/news-events/nih-research-matters/gene-therapy-eye-disease-shows-benefits-limitations
- https://www.nature.com/scitable/topicpage/gene-therapy-760
- https://link.springer.com/article/10.1007/s00417-012-2028-2
- https://www.asrs.org/patients/retinal-diseases/37/leber-congenital-amaurosis-lca
- https://www.nature.com/articles/s41434-020-00197-8
- https://luxturna.com
- https://www.drugs.com/mtm/luxturna.html
- https://www.fightingblindness.org/research/leber-congenital-amaurosis-research-advances-5
- https://www.ucsfhealth.org/conditions/leber-congenital-amaurosis-lca

Dr. Sumeet is a seasoned geneticist turned wellness educator and successful financial blogger. GenesWellness.com, leverages his rich academic background and passion for sharing knowledge online to demystify the role of genetics in wellness. His work is globally published and he is quoted on top health platforms like Medical News Today, Healthline, MDLinx, Verywell Mind, NCOA, and more. Using his unique mix of genetics expertise and digital fluency, Dr. Sumeet inspires readers toward healthier, more informed lifestyles.





